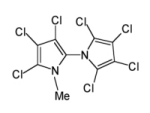
Figure: Structure of the natural product Q1
Systematic research in this field started in 1998, when we determined high concentrations of an unknown organochlorine compound we called Q1 in marine samples from Africa and the Antarctic (Mar. Poll. Bull. 38 (1999) 380-386). A concentrated sample was investigated with the help of high resolution mass spectrometry which resulted in the determination of the exact mass of Q1 being 383.814 Da which corresponds with the molecular formula C9H3Cl7N2 (Rapid Comm. Mass Spectrom. 21 (1999) 2118-2124). It is noteworthy that this molecular formula was previously not reported for a stable compound. Q1 was detected in different matrices such as human milk and air samples. In parallel we could show that Q1 is a persistent bioaccumulative halogenated natural product (Environ. Poll. 110 (2000) 401-409). Since Q1 was more abundant in marine samples from the southern hemisphere (Antarctic, southern Africa) than in samples from the northern hemisphere (Central and Northern Europe, North America), it appeared to be plausible to confirm this finding by the analysis of samples from Australia. In cooperation with Caroline Gaus and Jochen F. Müller (EnTox, Brisbane) we determined high concentrations in marine samples from the surrounding of Brisbane. The blubber of a dolphin from this region contained 14 ppm Q1 (!) which is the highest Q1 level determined to date (Arch. Environ. Contam. Toxicol. 41 (2001) 221-231, Chemosphere 46 (2002) 1477-1483).
Intensive mass spectrometric analyses indicated that Q1 possesses a bipyrrole backbone. We then synthesized Q1 in gram-amounts and elucidated the strucutre to be 2,3,3´,4,4´,5,5´-heptachloro-1´-methyl-1,2´-bipyrrole:

The synthesis of Q1 was finished independently at the same time in our lab and that of Gordon Gribble at Dartmouth College, and both groups shared the results in a joint publication (Angew. Chem. Intern. Ed. 41 (2002) 1740-1743). Q1 is the first 1,2´-bipyrrole derivative produced in the lab and it may sound unbelieveable but even the simple unsubstituted 1,2´-bipyrrole backbone was previously unknown (Article).
It is noteworthy that conventional samples (fish) are known from official food control in which Q1 was more abundant than all anthropogenic pollutants together (sum of PCBs, DDT and metabolites as well as other chlorinated pesticides and pollutants)!
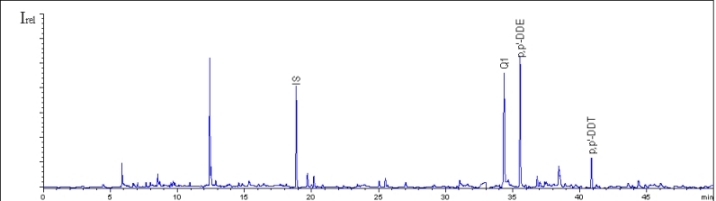
The following figure shows the chromatogram of a fish sample, in which the concentration of Q1 is only exceeded by p,p´-DDE.
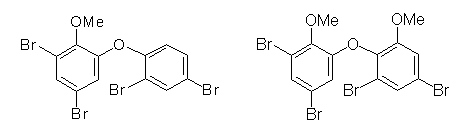
The samples from Australia not only accumulated high concentrations of Q1 but they also contained a number of uncommon abundant peaks which were identified as organobromine compounds (Arch. Environ. Contam. Toxicol. 41 (2001) 221-231). The prediction that these compounds are also halogenated natural products could be confirmed within short time, since around the same time, natural products chemist Mary Garson and coworkers (University of Queensland, Brisbane) determined several brominated natural products in sponges from the same region. Exchance of samples prove that two of the most important halogenated natural products in our samples were produced by sponges, and the structure of BC-2 was determined to be 4,6-dibromo-2-(2´,4´-dibromo)phenoxyanisole and BC-11 was found to be 3,5-dibromo-2-(3´,5´-dibromo, 2´-methoxy)phenoxyanisole (Environ. Toxicol. Chem. 21 (2002) 2014-2019).
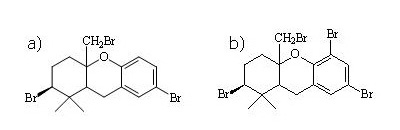
It is interesting that both brominated natural products differ only by one or two methoxy groups from the toxic brominated diphenyl ethers (BDEs), which are widely used as flame retardants (BILD). Just previoulsy the so-called pentabromo mixture was banned by the EU. In this context it is remarkable that the highest concentrations of natural organobromine compounds determined by us (ΣBCs >5 mg/kg lipid weight) are on the same level as highest concentrations of toxic BDEs ever determined in food and environmental samples (Environ. Toxicol. Chem. 21 (2002) 2014-2019). Therefore, it must be investigated whether the halogenated natural products show similar toxicity as anthropogenic BDEs and whether it is necessary to introduce residue limits in food for consumer´s protection.
Recently, we have detected several tribromophenoxyanisoles some of which have not yet been described by natural products chemists (Environ. Sci. Technol. 39 (2005) 7784). The substitution patterns of selected tribromo isomers suggested that they are metabolites of tetrabromophenoxyanisoles (Environ. Sci. Technol. 39 (2005) 7784).
BC-2 which has been mentioned previously was also synthesized (Chemosphere 52 (2003) 423-431) by us and - likewise Q1 - is now commercially available for quantitative analyses (standards of the synthesized natural products Q1 and BC-2 can be ordered from LGC Promochem.=>ordering numbers)
MHC-1 (mixed halogenated compound) was a further important halogenated natural product which could be investigated in cooperation with Josef Hiebl from Landesuntersuchungsamt für das Gesundheitswesen Südbayern in Oberschleißheim. He once detected a prominent peak in chromatograms of fish from the official food control and contacted us for cooperation due to our previous work on Q1. Again, the molecular formula of MHC-1 was determined with high resolution mass spectrometry to be C10H13Br2Cl3. Thus, MHC-1 is most likely a mixed halogenated monoterpene (Environ. Sci. Technol. 35 (2001) 4157-4162). MHC-1 was detected in fish samples from the official food control (pollack) with up to 0.9 mg/kg and was detected in marine samples from four continents.
In a cooperation with colleagues from the official German foodcontrol (Josef Hiebl, Oberschließheim) were were recentyl able to detect residues of a new class of halogenated natural products, which are aprticularly abundant in commercial fish from the Mediterranean Sea (J. Agric. Food Chem. 54 (2006) 2652-2657). These HNPs were identified for the first time by Mary Garson and co-workers in sponges from Australia collected in shallow water(Figure).
Fish farms are constructed close to the shoreline. This and the fact that commercial fish have higher lipid content has led to an increased abundance of these compounds in seafood. A permanent and intense control should therefore becarried out in order to pretect the consumer from undesired pollution.
Halogenated natural products are best detected with a GC/ECNI-MS method developed by us (Anal. Chem. 73 (2001) 4951-4957), which has been improved in the meantime (Fresenius Environ. Bull. 11 (2002) 170-175).
This high-sensitive method enabled detection of many other potential marine natural products in food (Eur. Food Res. Technol. 215 (2002) 523-528; Chemosphere 52 (2003) 415-422; Chemosphere 52 (2003) 423-431). One compound frequently detected in marine food is 2,4,6-tribromoanisole (TBA), which has both natural and anthropogenic sources.
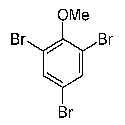
In nature, TBA is formed from tribromophenol, which is an important flavor compound of seafood (for instance seafish and shrimps). In cooperation with Martin Schlabach and Roland Kallenborn from the Norwegian Institute for Air Research (NILU) we identified TBA as an important brominated compound in air samples from both the Arctic and Antarctic (Fresenius Environ. Bull. 11 (2002) 170-175).
Our current investigations are focusing on the following topics: