(major congener in "Firemaster")
(BDE 209)
(BDE 47)
Identification of brominated natural products made it necessary to wide out our spectrum of anthropogenic pollutants with brominated flame retardants in order to distinguish them from brominated natural products as well as for comparisons of the respective concentrations of both types of brominated compounds in marine samples. In our view this is the best way of documenting the environmental relevance of both anthropogenic and natural brominated compounds.
The most important substance classes of nonpolar brominated flame retardants are polybrominated biphenyls (PBBs) and polybrominated diphenyl ethers (BDEs).
 |
 |
 |
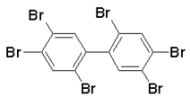
| Figure: structure of 2,2´,4,4´,5,5´-hexabrombiphenyl (major congener in "Firemaster") |
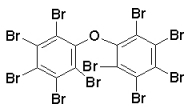
Figure: structure of decabromodiphenyl ether (BDE 209) |
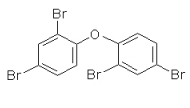
Figure: structure of 2,2´,4,4´-tetrabromodiphenyl ether (BDE 47) |
Next to the quantification of important BDE and PBB congeners in food we also investigate the role of axial chirality of PBBs (cooperation with Urs Berger and Espen Mariussen, NILU, as well as Arntraut Götsch - a former member of our group). HPLC with serially coupled C18-columns enabled isolation of more than 10 PBB congeners. Afterwards, it was possible to separate the enantiomers of the isolated PBBs using HPLC in combination with chiral stationary phases (J. Chromatogr. A 973 (2002) 123). Furthermore, we succeeded in enantioseparation of PBB 149, which has a molecular weight of over 600 Da, by enantioselective GC.
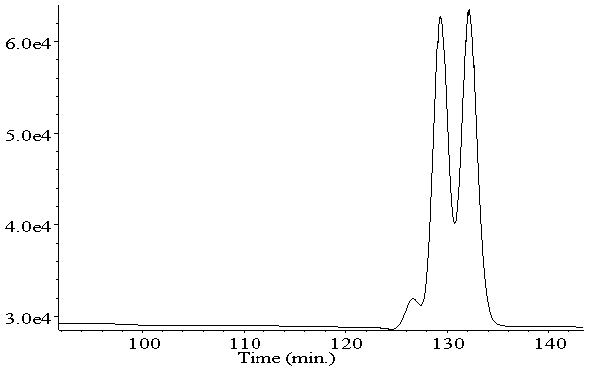
This offers us the possibility of studying the enantioselective degradation of environmentally relevant PBBs in food and environmental samples, where PBB 149 has already been identified.
In addition, we investigate the composition of technical BDE products und the anaerobic degradation of individual BDEs (cooperation with Ana Gago Martínez, María J. Nogueiras, and David Castejón of the institute of analytical and food chemistry at the university of Vigo in Spain).