Fatty acids are carboxylic acids with four carbons (butyric acid) or more. They are the central constituents of lipids and thus a fundamental ingredient of food. Fatty acids are mostly found esterificated with glycerin (1,2,3-trihydroxypropane, propanetriol) which yields the so-called triglycerides:
Next to the determination of the lipid content in foodstuff (which is listed on most food packages), the determination of individual fatty acids is an important task in food characterization.
Beyond the length of the chain (saturated fatty acids) fatty acids also differ in the degree of saturation (presence of double bonds, “monoenoic to hexaenoic fatty acids”). Unsaturated fatty acids may also vary in the position and configuration of double bonds (cis- and trans-fatty acids). When positions of double bonds need to be specified, this is done by starting at the end of the molecule. The position of the terminal carbon is labeled “n” or “omega” and positions and double bonds are then named n-3, n-6 , or wherever the terminal double bond is located. The corresponding fatty acids are summarized in so-called families (n-3 = omega-3, n-6, and n-9 families and so on). In the case of several double bonds, these are usually isolated which means that there is a sp2-hybrided carbon (a CH2 or methylene unit) lying between them. Low amounts of conjugated double bonds may also occur (conjugated linoleic acid, CLA). The possible spectrum of fatty acids in a food sample is further extended by branches (mainly in form of methyl groups), functional groups (for instance hydroxyl groups) or other substituents (for instance cyclopentyl).
The many potential structural features lead a high number of different fatty acids which can be found in food in varying amounts in food.
Palmitic, oleic, and linoleic acid (see Figure 1) usually dominate in food (typically found in single or to digit percentages), but these fatty acids are not the interesting ones in terms of nutritional or technological relevance where polyunsaturated (or polyenoic) acids and particularly n-3 fatty acids and CLA play a more important role.
Our interests are directed towards the analytical determination of fatty acids in food with special emphasis on branched-chain fatty acids.
Prior to the analysis of fatty acids lipids have to be isolated from other food constituents such as proteins and carbohydrates. We gain lipophilic components with the help of microwave-assisted extraction in open vessels (FOV-MAE) (Batista et al., 2001) or by accelerated solvent extraction (ASE). These fast and efficient techniques allow for substitution of time-consuming or tedious methods for lipid extraction such as the method according to Bligh and Dyer (for fish samples) and Soxhlet extraction. After evaporation of the solvent the extracted lipids are transesterificated and determined by GC/MS and GC/FID for the determination of the fatty acid profile. We apply different methods for the derivatization of fatty acids (preparation of fatty acid methyl esters = FAME or other derivatives) including reaction with BF3/MeOH, TMSH, and AMP. The GC-separation is mainly performed on stationary phases with high content of cyanopropyl subsitutents on the polysiloxane backbone.
Previous investigations have shown that food samples do often contain fatty acids which are not commercially available as reference standards. Thus, the GC determination of all fatty acids in a sample requires the application of GC/MS. GC/MS allows for the identification of co-elutions which are unavoidable irrespectively of the GC stationary phase used. This is possible by running FAME, which allow for the identification of the molecular ions (which also provides information on the number of double bonds, if present). However, information obtained from the mass spectra of FAME is limited since formation of the molecular ion does not only occur in the head group but also in the chain in the case of unsaturated fatty acids. This is accompanied by migration of double bonds due to mesomeric stabilization. Consequently, the initial positions of double bonds can not be unequivocally determined on the basis of the mass spectra of FAME. For the determination of positions of double bonds in fatty acids other derivatives should be used. Typical derivates are those where the carboxylic group of the fatty acid is transferred into a nitrogen-containing functional group („remote-site derivatization method“) in form of pyrrolidides, piperidyles, picolinylic esters, triazolepyridines, 2-alkenylbenzoxazoles, and 4,4-dimethyloxazolines. With this technique the molecular ion is almost exclusively located in the head group (the nitrogen and not the alkylic chain is baring the charge). This limits both the ion formation at double bonds and consequently migrations in the carbon chain. Therefore, the exact positions of double bonds and also branches can be assigned to the molecules. A detailed presentation of mass spectra of fatty acid derivatives was compiled by Christie.
Our strategy is based on a GC/MS-SIM method which enables a comprehensive determination of saturated (branched and long-chained) and unsaturated (monoenoic to hexaenoic) fatty acids in food, even when their contribution to the fatty acid pattern is very low. This is important since one of our major interests is directed to the appearance and relevance of branched-chain fatty acids in food.
As was shown in the chapter above, fatty acids are curently mainly determined by means of GC/FID. The disadvantages of this methodolgy are apparent: first, all organic compounds are detected with FID which thus is very non-selective; second, although the sensitivity of this detector is sufficient for the determination of major fatty acids, this technique limits the determination of minor fatty acids. If such minor fatty acids are to be determined, the possibilities of GC/FID are easily reached. Hence, our goal was the development of a detection method that allows for the secure simultaneous quantification of major- and minor fatty acids. That this was a task for GC/MS suggested itself. However, we were also aware that the common GC/MS full scan methods were insufficient. Therefore we attempted using GC/MS in the selected ion monitoring (SIM) mode which leads to improved sensitivity and reproducibility when quadrupoles are used. The question was, which derivates are most suited for the GC/MS-SIM-determination of fatty acids. Because of the almost endless number of theoretically possible fatty acids which, in dependence of chain-length, degree of saturation, and GC stationary phase, elute in the same elution range, chosing of the respective molecular ions in different time windows appeared pointless. Thus, our choice fell on methyl esters. Fig. 2 demonstrates that the mass spectra of fatty acids with the same chain length but different number of double bonds are very different (J. Agric. Food Chem. 53 (2005): 8896-8903).
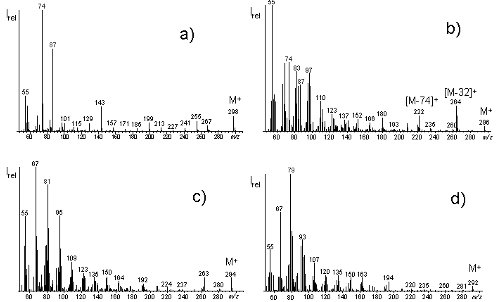
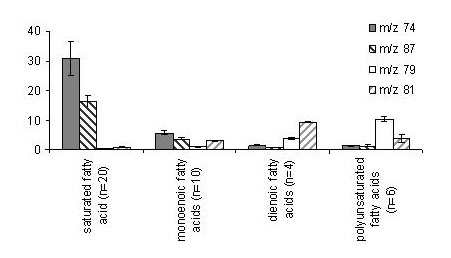
Saturated fatty acids generally show the McLafferty ion (m/z 74) as the most intense ion and m/z 87 which is formed via β-cleavage as second most abundant fragment ion (Fig. 3). The relevance of both fragment ions diminishes in the case of monoenoic fatty acids (Abb. 2b), although both fragment ions still are abundant in the mass spectra of this class of fatty acids. The situation s completely different for fatty acids with multiple double bonds. In the case of two (Fig. 2c) or >2 double bonds (Fig. 2d), m/z 74 and m/z 87 are virtually absent in the mass spectra (J. Agric. Food Chem. 53 (2005): 8896-8903).

On the other hand, in the high mass range neither the molecular ion nor other fragment ions were formed with sufficient abundance. However, all PUFA did form m/z 79 und m/z 81 as relatively characteristic fragment ions. Figure 4 shows, that the contribution of these fragment ions to the total ion current of FAME of the same class was mostly independent of the chain length.
GC/FID. However, the selectivity and sensitivity of the novel GC/MS-SIM method was much better compared with conventional GC/FID (J. Agric. Food Chem. 53 (2005): 8896-8903).
Methyl branches in fatty acids are typically found in n-1 positions (so-called iso-fatty acids) or in n-2 position (so-called anteiso-fatty acids):
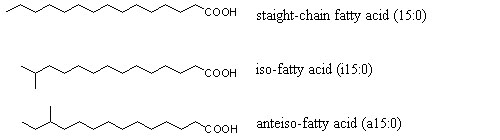
The nomenclature of branched-chain fatty acids (further branched-chain fatty acids) is based on the labeling of the longest straight chain in the molecule along with the position(s) and type of branching(s). For instance, the systematic name of isopentadecanoic acid (Figure 2) is 13-methyl-tetradecanoic acid (abbreviated i15:0) and that of the corresponding anteisopentadecanoic acid is 12-methyl-tetradecanoic acid (a15:0).
Humans consume about 0.5 g branched-chain fatty acids per day (compared to 8.54 g stearic acid, 18:0). Important sources are milk and milk products which may contain as much as 30 different branched-chain fatty acids. Low molecular branched chain fatty acids are partly responsible for the flavor of milk fat and are important contributors to the flavor of cheese. Human milk also contains over ten branched-chain fatty acids. Furthermore, they are found in human skin and epidermic lipids of diverse animals.
A series of animals and plants (beef, marine organisms, algae, tobacco, plant seems and oils of trees and many others) do contain branched chain fatty acids at 0.5 - 4 % of the lipid spectrum. This is on the same level as CLA and trans-fatty acids. However, they branched-chain fatty acids only investigated to a minor degree.
The presence of branched-chain fatty acids was often correlated with the presence of bacteria in a sample. This goes back to the fact that branched-chain fatty acids are found at very low concentrations in eukaryotes they may be dominating in prokaryotes. For instance, their contribution to the fatty acid spectrum of bacteria may be as much as 80 %.
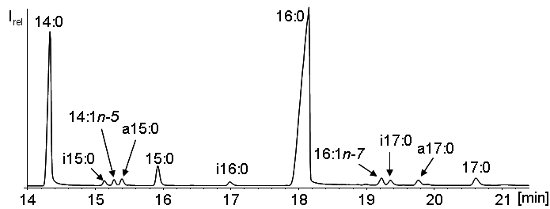
According to the known pathways of the biosynthesis of branched-chain fatty acids, mainly those with an odd carbon number and particularly iso- and anteiso-fatty acids with 15 and 17 carbons (i15:0, a15:0, i17:0, and a17:0) are formed. Isomeric fatty acids elute in the order iso-fatty acids < anteiso-fatty acids < straight-chain fatty acid (see Figure 3). Branched-chain fatty acids are also used as food additives for the preparation of cosmetics from synthetic materials.
Branching at the end of a fatty acid significantly changes the physicochemical properties such as the phase transfer temperature of lipids. Terminal branching increases the pressure and reduces the alteration of the enthalpy during the phase transfer. Therefore, iso- and anteiso-fatty acids are important for the membrane properties of lipids, since they disturb the orientation with similar consequences as double bonds.
On changing temperatures bacteria often react by alterations in their fatty acid composition in order to maintaining the lipid fluidity (homeoviscous adaption). This can be achieved by an increase in the amount of monoenoic fatty acids (bacteria generally do not produce polyenoic fatty acids), shortening of the average chain length and/or by an increased production of branched-chain fatty acids. For instance, it is known that the bacterium Listeria monocytogenes (which causes the foodborne disease listeriosis) does maintain its membrane fluidity when cooled (refridgerated) by an increased production of branched-chain fatty acids. Therefore, the pathogene is not killed by cooling or freezing of food and may even grow under these circumstances.
Due to their outstanding creep properties, their resistance against oxidation and their low melting points, branched chain fatty acids are also used in cosmetics.
Note that branched chain fatty acids and their transformation products are made responsible for the “flavor” and pheromonic effects of rams on female animals.
Enantioselective processes play an important and often fundamental role in nature. Many biosyntheses occur with high enantioselectivity. Although the relevance of chirality has been heavily documented for amino acids (mainly L-forms) and carbohydrates (mainly D-forms), only little scientific attention on this aspect has been paid to the third major group of food ingredients, lipids.
Lipids are often classified far too fast as nonchiral food ingredients. This is only partly true, since most of the triglycerides (all with R1 ? R3) and the phospholipids but also certain fatty acids are chiral.
Among the chiral fatty acids without an additional functional group, anteiso-fatty acids play the most important role in food.

Another main topic of our research is the enantioselective investigation of lipid constituents.
For investigations in this field we use a GC/MS- and a GC/FID system as well as an HPLC system.