
Figure: GC/ECD-chromatogram of toxaphene
Over many years, the complex chloropesticide "toxaphene" (camphechlor) has been an important part of our research. The technical mixture which has been used primarily in the 1970s has an application rate similar to DDT. It is composed of over 1000 components. Today, still less than 10% of them are structurally known.

Note that the major substance class in toxaphene, chlorinated bornanes, account for 16640 congeners and if one distinguishes enantiomers, there are 32767 individual compounds possible (Chemosphere 26 (1993) 1079-1086). In cooperation with Paul Andrews (formerly Health Canada) a nomenclature program was developed for chlorinated bornanes on the basis of IUPAC-rules. This program is available online on this homepage as free download (=>nomenclature system).
Toxaphene was mainly produced and used in the USA, but also in former USSR and GDR.
More interested readers may refer to the review article "Toxaphene. Analysis and Environmental Fate of Congeners", in "The Handbook of Environmental Chemstry (Volume 3, Part K: New Types of Persistent Halogenated Compounds Ed. J. Paasivirta, Springer Verlag, Berlin Heidelberg, 2000, pp. 237-287).
In the environment, many of the compounds of technical toxaphene (CTTs) are metabolized, and food samples contain much less toxaphene components and humans accumulate only two major products and some few minor compounds.
Since 1994, one of our major goals has been the elucidation of the structural pre-requisites for persistence of individual CTTs in higher organisms. For this reason, we developed new analytical methods and we isolated persistent CTTs and elucidated their structures (e. g. Environ. Sci. Technol. 31 (1997) 3023-3028; J. Agric. Food Chem. 49 (2001) 759-765). In cooperation with Gerd Scherer (Basel) the compounds were investigated with the help of molecular modeling (Environ. Sci. Technol. 33 (1999) 3458-3461). Today, we know the structure of eight of the eleven CTTs initially detected in Antarctic seals (Chromatographia 44 (1997) 65-73), six of which have been identified by our group (Focus article).
Since 1998 we also investigate processes, which play a role in the anaerobic degradation of toxaphene in soils and sediments. In a long-term cooperation with Keith Maruya from Skidaway Institute of Oceanography (Savannah/Georgia, USA) we also studied the transfer of individual CTTs from contaminated sediment into the food chain and followed their "destiny" in fish (Environ. Sci. Technol. 34 (2000) 1627-1635; Environ. Toxicol. Chem. 19 (2000) 2198-2203; Environ. Sci. Technol. 35 (2001) 4444-4448; Environ. Sci. Technol. 39 (2005) 3999-4004).
In parallel, we have established lab experiments in order to elucidate the degradation pathways of individual chlorinated bornanes. With this knowledge we want to develop strategies for elimination of toxaphene residues in the environment in such an effective way that as few CTTs as possible are then available for food chain enrichment, and thus reduce the chance that toxaphene will end up in our food.
First experiments were carried out with anaerobic sludge from a municipal sewage plant (Environ. Sci. Technol. 35 (2001) 960-965), but then we started a cooperation with microbiologist Anke Neumann (University of Karlsruhe) in order to use dehalogenating microorganisms. With the help of the anaerobic bacterium D. multivorans from the working group of Gabrielle Diekert (University of Jena) we were able to obtain good and promising successes. This bacterium enables the transformation of all persistent CTTs, which are accumulated by humans, into lower chlorinated CTTs. In turn, these transformation products can be easily degraded by humans and fish (Environ. Toxicol. Chem. 18 (1999) 2775-2781; Environ. Toxicol. Chem. 22 (2003) 2614-2621).
Vice versa, persistent CTTs and other organohalogen compounds in mammals are easily metabolized by S. multivorans (Toxicol. Environ. Chem. 87 (2005) 229-235).
The discovery of a corrinoid being the co-factor in the bacterium mentioned above initiated our research on the abiotic, anoxic transformation of toxaphene using commercially available corrinoids. When the central metal ion of the corrinoids - Co – is reduced to CoI with suitable agens such as titane(III)citrate, the resulting corrinoid is effictive in dehalogenating organohalogen compounds. Bacteria are "working" in a similar way. With the help of the superreduced corrinoids cyanocobalamin (CCAs, superreduced vitamin B12) and more effectively with dicyanocobinamide (DCCs) we observed an almost quantitative transformation of toxaphene within a couple of hours (see Figure) (Environ. Sci. Technol. 38 (2004) 3063-3067; Organohalogen Compd. 66 (2004) 2288-2293).
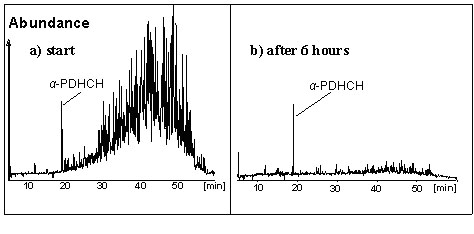
In additin, our interests are directed towards the elucidation the source of contaminations with toxaphene in food samples. We were able to show that the technical products Melipax® and Toxaphene® can be distinguished by using stable isotope mass spectrometry (IRMS) (Chemosphere 58 (2005) 235-241).